Design control is often a major source of confusion although; with time it has become more structured and better understood.
The design process is really the foundation of product quality, and just like a building if the foundation isn’t of sound construction the entire structure is at risk, or in the case of design the entire product.
Waterfall Model
The design control process not only applies to medical device products, but also should be applied to manufacturing processes and equipment, and an analogous system is used for software systems as well.
The waterfall method of design is depicted in the figure shown.
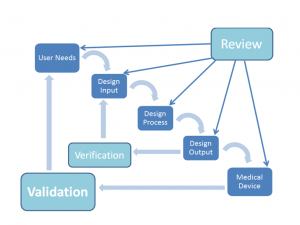
The waterfall diagram is the classic simplified view of the design process as depicted in the FDA’s Design Control Guidance.
Usually More Complex
The real design process is typically much more complicated as there are many elements which are being developed concurrently, but the waterfall diagram is a good basis for which to understand how the process is envisioned.
Design and Development – General: Common Mistake
One common mistake is to think of design control as the product development process.
While this is the core of design control it’s much more effective to view the product as a lifecycle approach see figure shown and realize that design control actually encompasses the entire lifecycle of the product.
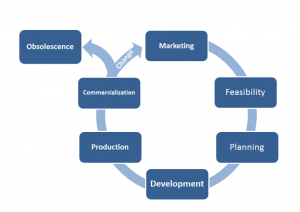
With that concept in mind it’s important to note that the design control process does not encompass any marketing requirements, or any feasibility requirements.
These phases are still critical to designing a marketable product but aren’t covered by the regulations because the regulations aren’t concerned with the success of the business only the safety and efficacy of the products that are put into the market place.
It is important not to mix up the marketing requirements and feasibility studies with the design input requirements.
These are also sometimes referred to as product concept documents.





