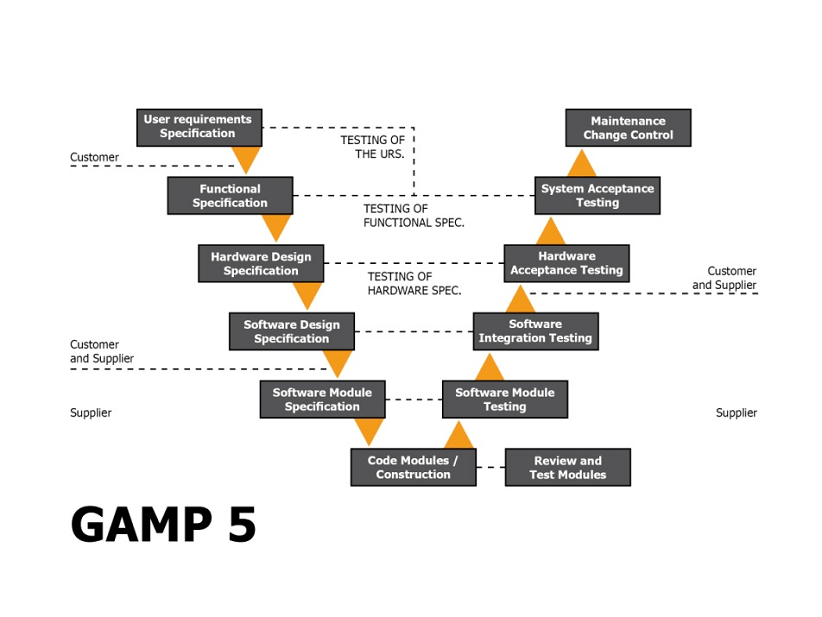
The new GAMP 5® A Risk Based Approach to Compliant GxP Computerized Systems provides a practical approach to achieving systems that are fit for their intended use in an efficient and highly effective manner, while also enabling advancement in technology and innovation. The improved GAMP® guide offers a more flexible risk based approach to compliant GxP systems based on scaleable specifications and verification. This approach relies heavily on having a robust quality risk management system. GAMP 5® also contains interesting information on out-sourcing electronic batch records and smaller spreadsheet and database applications.
The Life Science Industry is changing
The Life Science industry is responding to the challenge of an ever changing manufacturing environment, to incorporate new innovation in relation to drug development and manufacturing.
New concepts are being developed and deployed including science based risk management approaches and techniques. These approached are based on Quality by Design concepts.
As these news concepts and approaches are being developed the industry will for a period of time be in a state of transition.
As a result our thinking and strategies must also evolve to adapt to this new evolution. Hence the material we use to make these decisions must also change, that is why we now have GAMP 5® on the way.
Innovation is the Key
Where a computer system is regarded as one component of a wider manufacturing process or system, particularly in an integrated Quality by Design environment, specific and separate computerized system validation may not be necessary.
In such a case, the fitness for intended use of the computer system within the process may be adequately demonstrated by documented engineering or project activities together with subsequent Process Validation or continuous quality verification of the overall process or system.
While not all regulated companies will be in a position to fully embrace the new approaches immediately, GAMP 5 ® is intended to encourage the adoption of such approaches and in no way be a barrier.
Scope and Application
A wide and ever increasing range of local and global networked computerized systems are being used throughout the product life cycle of applications and systems. Many of these are subject to GxP activities.
Accuracy and integrity of records and data is essential throughout the product life cycle, from research and development through pre-clinical trials, production and quality control to marketing.
A Risk Based Approach to Compliant Electronic Records and Signatures provides further guidance on this topic, and should be used in conjunction with the GAMP 5® manual.
GAMP 5® aims to address the need to safeguard public health, product quality, and data integrity while at the same time enabling innovation and technology advance.
Patient Risk
By focusing on the risk to the patient and leveraging the expertise of the supplier and subject matter experts based on Good Engineering Practices, verification is considered as a previously called IQ and OQ. Regulated company IQ and OQ activities may then be omitted or limited to an assessment of the suppliers activities and documentation, and if necessary, performing mitigation activities to close gaps.
This eliminates much of the closely duplicated testing which does little or nothing to protect the plant.
The overall performance and fitness for intended purpose can be measured through Performance Qualification or Verification, which focus on critical-to-quality attributes.
Overall, this will demonstrate that the equipment of system process with which it is involved is controlled, and the risks to the patient have been effectively managed, thus meeting the regulatory requirements for validation.
Different Types of Computerized Systems
For integrated manufacturing systems or equipment where a computer-based system is part of the overall functionality, a specific and separate computerized system validation maybe required.
For example where the computer controlled equipment can be regarded as one component of a wider manufacturing or process control system the verification can be an integrated part of the overall process validation effort. The verification of fitness for intended use may be adequately demonstrated by documented integrated engineering or project activities together with subsequent Process Validation.
Validation is the common term used in regulations world-wide to describe a process that demonstrates that systems are fit for intended use. Some computerized systems are intimately involved in many regulated business activities outside the manufacturing area, and are critical for the health and protection of the patient.
Examples include the collection of clinical trial data, the management of donor details in blood collection, the recording of adverse events and complaints, the release of product for sale, and the recall of defective product.
Such IT systems have no direct correlation with the manufacturing and release of product. Consequently, there is no direct parallel with the manufacturing process and associated process validation. Acceptance of the system is dependent on the satisfactory completion of a functional test, such as the traditional OQ or equivalent tests, prior to a controlled cut over in to the live environment.
The principles of GAMP 5®
GAMP 5® describes a process which follows the following principles:
- The requirements of the system should be clearly defined.
- Requirements critical to the health and protection of the patient have been identified and the risks identified and controlled.
- The principles of GEP are applied throughout
- The testing carried out and documented by the supplier should be leveraged as much as possible.
- The critical to quality requirements are appropriately verified and reported by the regulated organization in line with regulatory expectations
- A complete system life cycle approach as part of a Quality Management System (QMS), from concept to retirement.
- A scaleable approach to achieve and maintain GxP compliance driven by novelty, complexity, and risk to patient safety, product quality, and data integrity.
- Clarifying the role of the Quality Unit, and introducing the roles of Process Owner, System Owner, and Subject Matter Experts.
- The leveraging of Supplier Documentation and Knowledge, wherever possible, and subject to satisfactory supplier assessment to avoid unnecessary duplication.
- Maximising the use of documentation from activities such as development and commissioning as verification evidence.
- Identifying opportunities for process and system improvements based on periodic review, root cause analysis, and corrective and preventative actions (CAPA).
It is also recommended that a plan describing and justifying the approach taken, and a report supporting that claim that the system is fit for intended use, are created.
Performed in this way, the process described above for computerized systems meets all of the GxP regulatory expectations for vaslidation.